
- About MicuRx
- Scientific research
- Pipeline
- Newsroom

Pipeline
- Home
- Pipeline

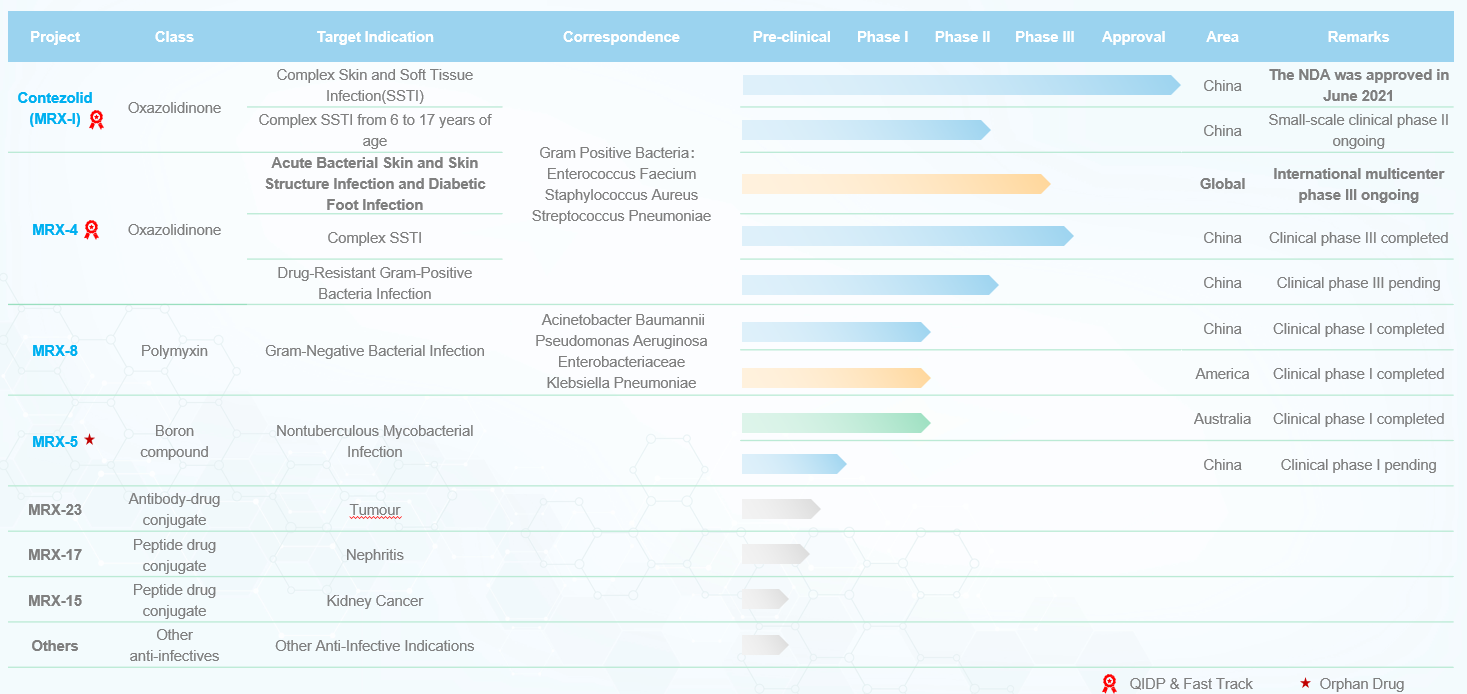
MicuRx is dedicated to creating treatments for patients with infections caused by multidrug-resistant super bacteria. Beyond that, MicuRx also has a pipeline of other investigational medicines for cancer and inflammation.
-
Contezolid (MRX-I) MRX-I
1、Next generation oxazolidinone antibiotic designed and developed by MicuRx
2、Target multidrug-resistant Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus,vancomycin-resistant Enterococci, penicillin-resistant Streptococcus pneumoniae, etc
3、Supported by National Major Scientific and Technological Special Project for “Significant New Drugs Development” during 11th, 12th, 13th five-year period4、Global Multi-Center Phase III trial initiated (MRX-4 sequential Contezolid)
- Patent filled in 2008
- China IND approved in 2010
- QIDP and Fast Track granted by US FDA by 2018
- China Phase 3 trial completed in 2019
- NDA granted priority review by NMPA in 2020
- NDA approved by NMPA in 2021
-
MRX-4 MRX-4
1、Injectable new oxazolidinone antibiotics designed and developed by MicuRx
2、Target multidrug-resistant Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus,vancomycin-resistant Enterococci, penicillin-resistant Streptococcus pneumoniae, etc
3、Supported by National Major Scientific and Technological Special Project for “Significant New Drugs Development” during 13th five-year period4、Global Multi-Center Phase III trial initiated (MRX-4 sequential Contezolid)
- Patent filled in 2015
- US Phase 1 trial completed in 2017
- QIDP and Fast Track granted by US FDA by 2018
- US Phase 2 trial completed in 2019
- China Phase 1 trial completed in 2021
- China Phase 3 trial completed in 2024
-
MRX-8 MRX-8
1、Next generation polymyxin antibiotic designed and developed by MicuRx
2、Target multidrug-resistant Gram-negative bacteria, including carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa, carbapenem-resistant Klebsiella pneumoniae, etc
3、Supported by National Major Scientific and Technological Special Project for “Significant New Drugs Development” during 13th five-year period- Patent filled in 2015
- US IND approved in 2020
- US Phase I trail completed in 2022
- Chinese Phase I trail completed in 2024
-
MRX-5 MRX-5
1、Novel boron compound designed and developed by MicuRx
2、Target non-tuberculous Mycobacteria infection- Australian Phase I trail completed in 2024
-
MRX-17 MRX-17
1、Novel kidney-targeted treatment designed and developed by MicuRx
2、To achieve superior efficacy and safety by increasing the local concentration of active drug via renal-targeted delivery -
MRX-23 MRX-23
A next-generation monoclonal antibody drug conjugate (ADC) targeting tumors
Featuring a new generation of camptothecin toxin and an optimized linker
-
MRX-15 MRX-15
1、Novel kidney-targeted treatment designed and developed by MicuRx
2、To achieve superior efficacy and safety by increasing the local concentration of drug via renal-targeted delivery
- Scientific research